*Patients were randomized 1:1 to receive ERLEADA® 240 mg orally once daily (n=525) or placebo orally once daily (n=527).1,3
†Patients receiving ERLEADA® should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy.1
NEW OS RWE: See head-to-head comparison of ERLEADA® vs enzalutamide and vs abiraterone acetate below
Extends OS for patients with mCSPC or nmCRPC1-6
Efficacy in the TITAN study
Dual primary endpoint, final analysis
ERLEADA® + ADT DEMONSTRATED SUPERIOR OS IN PATIENTS WITH mCSPC VS ADT ALONE1,2,4,7
Kaplan-Meier Analysis of OS (TITAN)1,2

Median follow-up time was 44.0 months.1
The survival rate at 48 months was 65.1% for ERLEADA® + ADT patients vs 51.8% for placebo + ADT patients.1 OS improved regardless of Gleason score, age, high- or low-disease volume, and metastasis stage at diagnosis. The TITAN primary analysis results: Median OS: NE vs NE; HR=0.67; 95% CI: 0.51, 0.89; P=0.0053. Median follow-up time was 22.7 months.1,2
Review the final OS results of the TITAN study
published in the Journal of Clinical Oncology
Dual primary endpoint, primary analysis
ERLEADA® + ADT DEMONSTRATED SUPERIOR rPFS VS ADT ALONE1,3,4

rPFS in Intent-to-Treat mCSPC Population (TITAN)1

Median follow-up time was 22.7 months.3
ERLEADA® + ADT improved rPFS vs placebo + ADT in a range of patient types with mCSPC.2,4 rPFS improved regardless of high- or low-volume disease, prior docetaxel use, or Gleason score at diagnosis.3
Efficacy in the SPARTAN study
Primary endpoint, primary analysis
ERLEADA® + ADT DEMONSTRATED A 2-YEAR IMPROVEMENT IN MEDIAN MFS VS ADT ALONE1,6
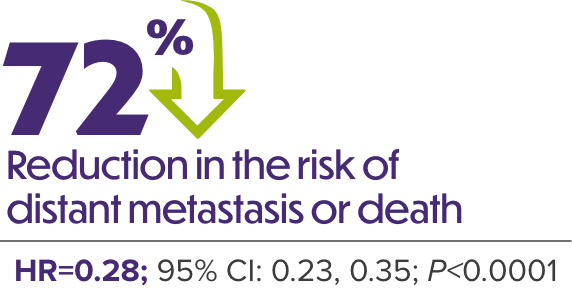
MFS in Patients With nmCRPC (SPARTAN)

Median follow-up was 20.3 months.6
Secondary endpoint, final analysis
ERLEADA® + ADT SIGNIFICANTLY IMPROVED OS IN PATIENTS WITH nmCRPC VS ADT ALONE1,5
OS in Patients With nmCRPC (SPARTAN)

Median follow-up was 52.0 months.5
Review the final OS results of the SPARTAN study published in European Urology
See the data
IMPORTANCE OF PSA EXPLORATORY DATA FROM THE TITAN AND SPARTAN CLINICAL TRIALS AND REAL-WORLD DATA
Limitations: These data are from exploratory endpoints in clinical trials, post hoc analyses, and real-world studies and are not included in the full ERLEADA® Prescribing Information. PSA should be viewed in the context of patient management and the overall physical condition and clinical course of the patient. PSA responses are not reported in product labeling because they are not validated biomarkers.
Efficacy in the TITAN study
TIME TO PSA PROGRESSION: ERLEADA® + ADT VS ADT ALONE

Median follow-up of 44.0 months.2
‡Time to PSA progression was defined as the time from randomization to PSA progression, according to Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria. For patients who experienced a decline in PSA from baseline, PSA progression was defined as the first PSA increase that was ≥25% and ≥2 ng/mL above the nadir, which was confirmed by a second value obtained 3 or more weeks later. For patients who did not experience a decline in PSA from baseline, PSA progression was defined as a PSA increase that was ≥25% and ≥2 ng/mL above the baseline value after 12 weeks.2,10
Time to PSA progression and PSA response rate are not reported in product labeling because they are not validated biomarkers. PSA evaluation should be viewed in the context of patient management and the overall physical condition and clinical course of the patient.11
PSA Response at 3 Months

PSA Response at 12 Months

PSA-OS Relationship in TITAN: PSA90 With ERLEADA® + ADT

PSA-OS Relationship in TITAN: PSA Undetectable With ERLEADA® + ADT

Consistent outcomes in the real world13 and TITAN2

Data Source13:
Flatiron Metastatic Prostate Cancer Core Registry Electronic DataMart (1/1/2013 to 7/31/2022)
Limitations13:
Analyses were descriptive only; no adjustments were made for potential baseline confounders
- Flatiron represents the oncology setting and may not be representative of the entire population of ERLEADA® initiators in the United States
- Study relied upon clinical data that may be subject to misclassification or reporting inaccuracies
- No formal comparisons between agents were conducted
Study Design13:
Descriptive, retrospective, longitudinal cohort design
- Patients with metastatic disease without evidence of CR before initiating ARSI were classified into 3 cohorts: ERLEADA®, enzalutamide, or abiraterone acetate
- CR was identified by a Flatiron algorithm incorporating:
- Physician-reported CR in medical chart
- Rising PSA value of ≥2 ng/mL while on hormone therapy and ≥1 higher subsequent PSA value within 3 months of first elevated PSA testOR
- Physician-documented rising PSA on hormone therapy plus a change in therapy
- Concurrent use of ADT was not required; concurrent use of prednisone was not required for the abiraterone acetate cohort
- History of ADT use was observed in 83.2% of patients in the ERLEADA® cohort
§Overall survival rate at 24 months in TITAN derived from Kaplan-Meier estimates of overall survival from Chi KM, et al. (Figure 1A).2
Primary endpoint: OS at 24 months

Comparision Of Overall Survival Among Patients With mCSPC



There have been no prospective head-to-head trials comparing the safety or efficacy of ERLEADA® to enzalutamide. This was a causal head-to-head analysis intended to evaluate and compare the effects of treatments on overall survival by 24 months.
Clinical real-world data collected from community urology practices were linked with administrative claims data in the United States (study period: December 16, 2018, to December 31, 2023). Overall survival at 24 months was evaluated in a retrospective, longitudinal propensity score–weighted causal analysis of patients with mCSPC who were treated with ERLEADA® (n=1,810) or enzalutamide (n=1,909).9
Limitations9:
- Precision Point Specialty (PPS) represents the community urology setting and may not be representative of the entire mCSPC population in the United States
- Miscoding or misclassification in clinical records or administrative claims can lead to selection and limitation biases despite efforts to balance the study populations
- While the Komodo Research Database captured >90% of oncology-related deaths, as validated against CDC estimates, some deaths may still be missed
- Regression analyses could only adjust for documented covariates, and unknown confounders may be present
- Unlike Phase 3 trials assessing overall survival at specific events, this study evaluated survival at 24 months. Longer follow-up studies may be needed to fully assess the therapeutic effects
||This real-world analysis relied on clinical data that may contain inaccuracies or omissions (eg, diagnosis dates, treatment start dates, and other variables) and does not capture any diagnoses, medical services, or prescription fills obtained outside of the urology practice. The database represents the community urology setting and might not be representative of the entire population of ERLEADA® initiators in the United States, which may limit the generalizability of the study.9
¶Propensity scores were generated using probability estimates from logistic regression models using the following predictors: age (continuous), race, geographic region, payer, year of index date, time between metastasis and index date (continuous and categorical), time between PC diagnosis and index date (continuous), de novo PC, ADT use overlapping with index date, first-generation anti-androgen use, chemotherapy use, types of metastases (bone, nodal, visceral, and metastasis in multiple sites), Quan-Charlson comorbidity index (continuous), most recent PSA level (categorical), and earliest Gleason score (categorical). Each patient was attributed an inverse-probability of treatment weight that was defined as follows: 1/(propensity score) for the ERLEADA® cohort and 1/(1-propensity score) for the enzalutamide cohort. Normalized inverse-probability of treatment weights were truncated at the 95th percentile.9
#A hazard ratio <1 indicates that the ERLEADA® cohort had a lower rate of death compared with the enzalutamide cohort.9
**Significant at the 5% level.9
††Of note, the number of patients reported in this weighted population represents the sum of weights for the corresponding nonweighted patients, rounded to the nearest integer. The proportions displayed were calculated before the rounding and may be slightly different than if they were calculated based on rounded numbers.9
Primary endpoint: OS at 24 months

Comparision Of Overall Survival Among Patients With mCSPC



There have been no prospective head-to-head trials comparing the safety or efficacy of ERLEADA® to abiraterone acetate. This was a causal head-to-head analysis intended to evaluate and compare the effects of treatments on overall survival by 24 months.
Clinical real-world data collected from community urology practices were linked with administrative claims data in the United States (study period: September 17, 2018, to December 31, 2023). Overall survival at 24 months was evaluated in a retrospective, longitudinal propensity score–weighted causal analysis of patients with mCSPC who were treated with ERLEADA® (n=1,897) or abiraterone acetate (n=2,073).13
Limitations13:
- PPS represents the community urology setting and may not be representative of the entire mCSPC population in the United States
- Miscoding or misclassification in clinical records or administrative claims can lead to selection and limitation biases despite efforts to balance the study populations
- While the Komodo Research Database captured >90% of oncology-related deaths, as validated against CDC estimates, some deaths may still be missed
- Abiraterone acetate is only indicated for high-risk mCSPC, which may result in residual differences relative to the ERLEADA® treatment cohort after IPTW adjustments
- Regression analyses could only adjust for documented covariates, and unknown confounders may be present
- Unlike Phase 3 trials assessing overall survival at specific events, this study evaluated survival at 24 months. Longer follow-up studies may be needed to fully assess the therapeutic effects
‡‡This real-world analysis relied on clinical data that may contain inaccuracies or omissions (eg, diagnosis dates, treatment start dates, and other variables) and does not capture any diagnoses, medical services, or prescription fills obtained outside of the urology practice. The database represents the community urology setting and might not be representative of the entire population of ERLEADA® initiators in the United States, which may limit the generalizability of the study.13
§§Propensity scores were generated using probability estimates from logistic regression models using the following predictors: age (continuous), race, geographic region, payer, year of index date, time between metastasis and index date (continuous and categorical), time between PC diagnosis and index date (continuous), de novo PC, ADT use overlapping with index date, first-generation anti-androgen use, chemotherapy use, types of metastases (bone, nodal, visceral, and metastasis in multiple sites), Quan-Charlson comorbidity index (continuous), most recent PSA level (categorical), and earliest Gleason score (categorical). Each patient was attributed an inverse-probability of treatment weight that was defined as follows: 1/(propensity score) for the ERLEADA® cohort and 1/(1-propensity score) for the abiraterone acetate cohort. Normalized inverse-probability of treatment weights were truncated at the 95th percentile.13
||||A hazard ratio <1 indicates that the ERLEADA® cohort had a lower rate of death compared with the abiraterone acetate cohort.13
¶¶Significant at the 5% level.13
##Of note, the number of patients reported in this weighted population represents the sum of weights for the corresponding nonweighted patients, rounded to the nearest integer. The proportions displayed were calculated before the rounding and may be slightly different than if they were calculated based on rounded numbers.13
REAL-WORLD PSA50 AND PSA90 RESPONSES WERE SIMILAR TO THOSE SEEN IN THE TITAN STUDY
| PSA50 | PSA90 | |
|---|---|---|
| % of mCSPC patients who achieved PSA responses | ||
| Real-world Clinical Data (>8 Weeks) | 86.7% | 73.5% |
| TITAN (At Any Time) mCSPC | 90% | 72% |
Clinical real-world data collected as part of routine clinical care from 69 urology sites in the United States (study period: February 2017 - March 5, 2021). PSA responses to ERLEADA® were observed in real-world data >8 weeks after initiation of ERLEADA® and in the TITAN study at any time after starting treatment. Responses were evaluated in a retrospective longitudinal cohort study of patients with mCSPC (n=260) or nmCRPC (n=313) who were treated with ERLEADA®.14
***This real-world analysis relied on clinical data that may contain inaccuracies or omissions (eg, diagnosis dates, treatment start dates, and other variables) and does not capture any diagnoses, medical services, or prescription fills obtained outside of the urology practice. The database represents the community urology setting and might not be representative of the entire population of ERLEADA® initiators in the United States, which may limit the generalizability of the study.14
PSA RESPONSE IN BLACK AND NON-BLACK PATIENTS TREATED WITH ERLEADA® WAS CONSISTENT WITH SPARTAN STUDY DATA
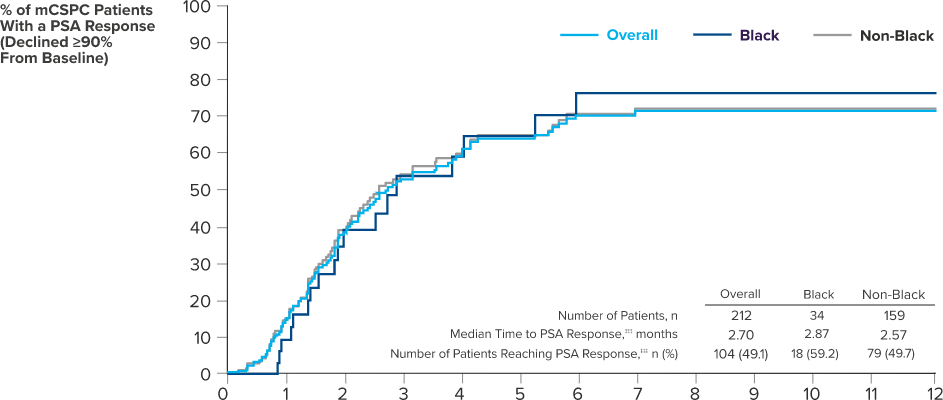
Clinical real-world data collected as part of routine clinical care from 69 urology sites in the United States (study period: February 2017 - March 5, 2021). PSA responses to ERLEADA® were observed in real-world data >8 weeks after initiation of ERLEADA® and in the SPARTAN study at any time after starting treatment. Responses were evaluated in a retrospective longitudinal cohort study of patients with mCSPC (n=260) or nmCRPC (n=313) who were treated with ERLEADA®.14
†††This real-world analysis relied on clinical data that may contain inaccuracies or omissions (eg, diagnosis dates, treatment start dates, and other variables) and does not capture any diagnoses, medical services, or prescription fills obtained outside of the urology practice. The database represents the community urology setting and might not be representative of the entire population of ERLEADA® initiators in the United States, which may limit the generalizability of the study.14
‡‡‡PSA response was defined as the first decline for a follow-up PSA value of 90% or more from the most recent PSA value within 13 weeks of the index date.14
IMPORTANCE OF PSA EXPLORATORY DATA FROM THE TITAN AND SPARTAN CLINICAL TRIALS AND REAL-WORLD DATA
Limitations: These data are from exploratory endpoints in clinical trials, post hoc analyses, and real-world studies and are not included in the full ERLEADA® Prescribing Information. PSA should be viewed in the context of patient management and the overall physical condition and clinical course of the patient. PSA responses are not reported in product labeling because they are not validated biomarkers.
Efficacy in the SPARTAN study
TIME TO PSA PROGRESSION: ERLEADA® + ADT VS ADT ALONE

Median follow-up time was 52.0 months.5
‡Time to PSA progression: Median NE vs 3.7 months; HR=0.07; 95% CI: 0.06, 0.09.5
§Time to PSA progression was defined as the time from randomization to PSA progression, according to Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria. For patients who experienced a decline in PSA from baseline, PSA progression was defined as the first PSA increase that was ≥25% and ≥2 ng/mL above the nadir, which was confirmed by a second value obtained 3 or more weeks later. For patients who did not experience a decline in PSA from baseline, PSA progression was defined as a PSA increase that was ≥25% and ≥2 ng/mL above the baseline value after 12 weeks.5,10
PSA response: 38% of patients in the ERLEADA® + ADT arm achieved a confirmed PSA level ≤0.2 ng/mL vs no patients in the placebo + ADT arm.5
Time to PSA progression and PSA response rate are not reported in product labeling because they are not validated biomarkers. PSA evaluation should be viewed in the context of patient management and the overall physical condition and clinical course of the patient.11
PSA Response at 3 Months

PSA Response at 12 Months

PSA-OS Relationship in SPARTAN: PSA90 With ERLEADA® + ADT

PSA-OS Relationship in SPARTAN: PSA Undetectable With ERLEADA® + ADT

REAL-WORLD PSA50 AND PSA90 RESPONSES WERE SIMILAR TO THOSE SEEN IN THE SPARTAN STUDY
| PSA50 | PSA90 | |
|---|---|---|
| % of nmCRPC patients who achieved PSA responses | ||
| Real-world Clinical Data (>8 Weeks) | 82.3% | 66.0% |
| SPARTAN (At Any Time) nmCRPC | 90% | 62% |
Clinical real-world data collected as part of routine clinical care from 69 urology sites in the United States (study period: February 2017 - March 5, 2021). PSA responses to ERLEADA® were observed in real-world data >8 weeks after initiation of ERLEADA® and in the SPARTAN study at any time after starting treatment. Responses were evaluated in a retrospective longitudinal cohort study of patients with mCSPC (n=260) or nmCRPC (n=313) who were treated with ERLEADA®.14
||This real-world analysis relied on clinical data that may contain inaccuracies or omissions (eg, diagnosis dates, treatment start dates, and other variables) and does not capture any diagnoses, medical services, or prescription fills obtained outside of the urology practice. The data base represents the community urology setting and might not be representative of the entire population of ERLEADA® initiators in the United States, which may limit the generalizability of the study.14
PSA RESPONSE IN BLACK AND NON-BLACK PATIENTS TREATED WITH ERLEADA® WAS CONSISTENT WITH SPARTAN STUDY DATA

Clinical real-world data collected as part of routine clinical care from 69 urology sites in the United States (study period: February 2017 - March 5, 2021). PSA responses to ERLEADA® were observed in real-world data >8 weeks after initiation of ERLEADA® and in the SPARTAN study at any time after starting treatment. Responses were evaluated in a retrospective longitudinal cohort study of patients with mCSPC (n=260) or nmCRPC (n=313) who were treated with ERLEADA®.14
¶This real-world analysis relied on clinical data that may contain inaccuracies or omissions (eg, diagnosis dates, treatment start dates, and other variables) and does not capture any diagnoses, medical services, or prescription fills obtained outside of the urology practice. The database represents the community urology setting and might not be representative of the entire population of ERLEADA®initiators in the United States, which may limit the generalizability of the study.14
#PSA response was defined as the first decline for a follow-up PSA value of 90% or more from the most recent PSA value within 13 weeks of the index date.14
Established safety profiles in the TITAN and SPARTAN studies
ADT, androgen deprivation therapy; CDC, Centers for Disease Control and Prevention; CI, confidence interval; GnRH, gonadotropin-releasing hormone; HR, hazard ratio; mCSPC, metastatic castration-sensitive prostate cancer; MFS, metastasis-free survival; NE, not estimable; nmCRPC, non-metastatic castration-resistant prostate cancer; NR, not reached; OS, overall survival; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival; SPARTAN, Selective Prostate Androgen Receptor Targeting with ARN-509; TITAN, Targeted Investigational Treatment Analysis of Novel Anti-androgen.
REFERENCES:
- ERLEADA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294-2303.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24.
- A study of apalutamide (JNJ-56021927, ARN-509) plus androgen deprivation therapy (ADT) versus ADT in participants with mCSPC (TITAN). ClinicalTrials.gov identifier: NCT02489318. Updated January 3, 2025. Accessed June 3, 2025. https://clinicaltrials.gov/study/NCT02489318
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150-158.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. Supplement. N Engl J Med. 2019;381(1):13-24. doi:suppl/10.1056/NEJMoa1903307/suppl_file/nejmoa1903307_appendix.pdf
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.4.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed June 17, 2024. To view the most recent and complete version of the guideline, go to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Bilen MA, Lowentritt B, Khilfeh I, et al. Overall survival with apalutamide versus enzalutamide in metastatic castration-sensitive prostate cancer. Adv Ther. 2025. doi:10.1007/s12325-025-03207-6
- Scher HI, Halabi S, Tannock I, et al; Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159.
- Crawford ED, Bennett CL, Andriole GL, Garnick MB, Petrylak DP. The utility of prostate-specific antigen in the management of advanced prostate cancer. BJU Int. 2013;112(5):548-560.
- Chi KN, Saad F, Chowdhury S, et al. Prostate-specific antigen kinetics in patients with advanced prostate cancer treated with apalutamide: results from the TITAN and SPARTAN studies. Poster presented at: American Urological Association Annual Meeting; September 10-13, 2021; Las Vegas, Nevada.
- Lowentritt B, Bilen MA, Khilfeh I, et al. Overall survival in patients with metastatic castration-sensitive prostate cancer treated with apalutamide versus abiraterone acetate: a head-to-head analysis of real-world patients in the USA. J Comp Eff Res. 2025. doi:10.57264/cer-2025-0023
- Bivins VM, Durkin M, Khilfeh I, et al. Early prostate-specific antigen response among Black and non-Black patients with advanced prostate cancer treated with apalutamide. Future Oncol. 2022;18(32):3595-3607.



